| |
 |
| |
 |
| (1) Structure and Layout of the Heating Furnaces |
| (2) Material's Viewpoints, Radiation, Transfer, and Conduction of Heat |
| Efficiency of the Furnace |
| (3) How to Simulate the Temperature |
| Thermal Radiation |
| Heat Transfer |
| Heat Conduction |
| |
 |
| (1) Structure and Layout of the Heating Furnaces |
| |
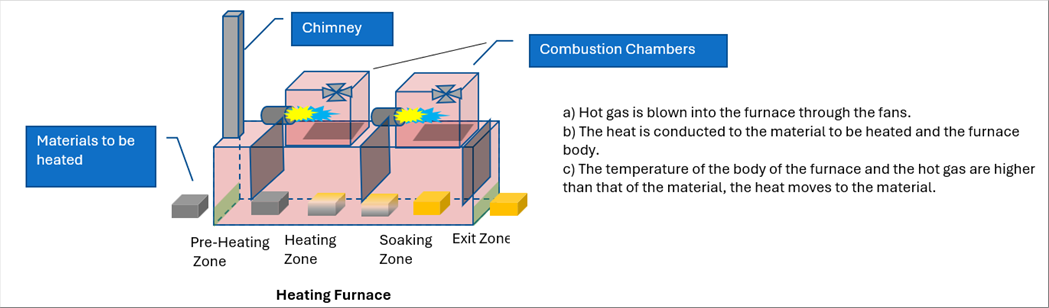
| One of the ways of heating is to use the Heating Furnace. The Heating Furnace is made with the heat insulation materials. It is to heat the material by the combustion of the flammable gas, which is at either in hot or in the room temperature. The following picture shows an example of the heating furnaces, which is equipped with the combustion chambers on the top. It is to burn the flammable gas in the combustion chamber. The hot combustion gas is blown into the furnace through the fans. The material to be heated enters into the from the right and comes out of the furnace to the right. |
| |
| There are three (3) partition plates in the picture. By the plates, there are four (4) zones inside of the furnace, which are pre-heating, heating, soaking, and exit ones. Thanks to the plates, the temperature control of each zone is easier than those with no plates. Please note that they are not mandatory. |
| |
 |
| |
| There is a chimney on the right end of the pre-heating zone. It is because the combustion gas is designed flowing from the right to the left of the furnace. |
| |
| Thanks to the design, the combustion gas gan be supplied from the higher temperature areas to the pre-heating zone without burning equipment on the zone. It is good for the energy conservation. |
| |
| The picture does not indicate the pressures; however, the gas pressure in the furnace is controlled bit higher than that of the external air. It is very important for the prevention of temperature drop in the furnace. The higher pressure in the furnace prevents the invasion of the air into the furnace. If the outside air touches the material in the furnace, the temperature of the point becomes lower, but no invasion, no impact. |
| |
| The outside air can invade into the furcate at the entry and the exit points of the furnace. In the picture, the pre-heating and exit zones are seen in order to minimize the impact of the invasion. |
| |
 |
| (2) Material's Viewpoints, Radiation, Transfer, and Conduction of Heat |
| |
| In the section (1), we looked at the furnace from the viewpoint of the mechanism. Let's see it from the viewpoint how the temperature of the material rises. One of the ways is to put the viewpoint on the material. |
| |
| Please imagine that the materal can "see". It recieves energy from all areas, which it can "see". |
| |
| The material see the plates, which devide the furnace into 4 zones as it moves. It can sense the temperature of all the zones are higher than itself. The temperature becomes higher and higher as it moves. It realizes the reason why the temperature of it becomes higher and higher. |
| |
| Now, lt's think about the heat and the temperature. |
| |
| First of all, the definitions are as following. The heat is an energy, and the temperature is the index of the magnitude of the heat energy. The lower temperature area contains fewer heat energy, the high temperature one, more heat energy. In the usual story, the heat energy is simply stated as heat. |
| |
| The direction of the heat is one way, it flows from the higher temperature area to the lower one, no opposite direction. |
| |
| Therefore, the heat always moves from the higher temperature area to the lower one. |
| |
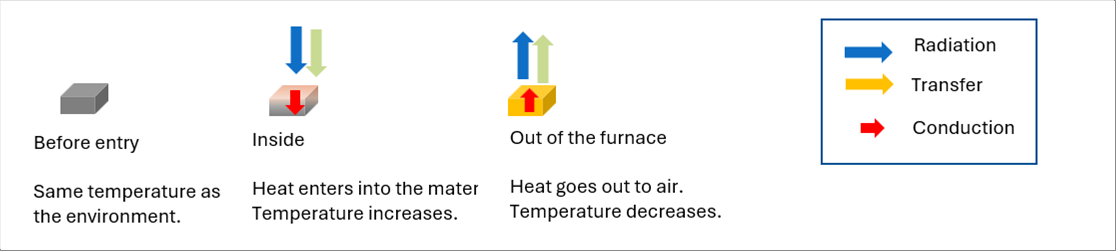
| The temperature of the material is equal to the one of the outside air. The heats enters into the material when it enters into the furnace. The heat come out of the material when it comes out of the furnace. (If the temperature of sone areas of the furnace is lower than that of the material, the heat comes out of the material to the furnace, though.) |
| |
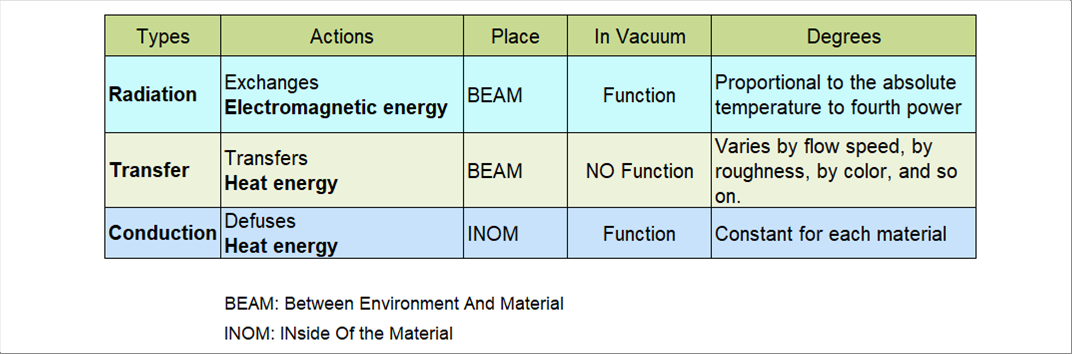
| There are three (3) types of the heat movement, which are thermal radiation, heat transfer, and the heat conductivity. |
| |
 |
| |
| Both of the heat radiation and the heat transfer are heat moves between the surrounding areas, and the heat conductivity is inside of the material. |
| |
| Both of the heat radiation and the heat conductivity work in vacuum. However, the heat transfer work only with the fluid like water and air. |
| |
 |
| |
 |
| Efficiency of the Furnace |
| |
| It may be sometimes necessary to heat the material as fast as possible without any cost; however, in general circumstances, the minimum fuel consumption is required with the defined time and temperature.. |
| |
| The total calorific value is used to the temperature increases of the three (3) items, which are combustion fluid (both of fuel and air), furnace body, and the material. The remaining one, which is not used for the 3 items, goes out of the furnace through the chimney. |
| |
| Therefore, the efficiency how much percentage (%) of the total calorific value enters into the material. In other word, the lower temperature of the chimney gas, the higher efficiency. |
| |
 |
| (3) How to Simulate the Temperature |
| |
| it is necessary for the temperature simulation to calculate the three (3) items, which are heat radiation, the heat transfer and the heat conduction. The first two (2) items gives the incoming heat to the material, and the third one how the heat moves inside of the material. |
| |
 |
| |
 |
| Blue: Heat Radiation |
| |
| Heat radiation is always happening. It stops at the absolute temperature zero (0) degrees. The radiation can reach the material as long as the material can "see" it. What it sees changes from the entry to the exit of the furnace. The shape factor is important what percentage of the radiation reaches it. The heat radiation is calculated by the radiation flux multiplied by the shape factor. (Please see the thermodynamics theory for detail.) |
| |
 |
| Orange: Heat Transfer |
| |
| When a material with the different temperature than the environment, the air around the material starts flowing. If the material is hotter than the air, the air lows upward, and vise versa. The bigger difference of the temperatures, the faster flow speed. It is necessary to calculate the air flow speed by the flow dynamics. Then, the heat transfer coefficient can be obtained. (Please refer to the flow dynamics and the thermodynamics,.) |
| |
 |
| Red: Heat Conduction |
| |
| Once the heat is transmitted to inside of the material, we can only focus on the heat conductivity coeffficient, which is unique to each material. (Pleare refer to the theory of the thermo dynamics. |
| |
| Author: T. Oda |
| Preparation in Excel, automatic html / css generation by the "excel2web". |

























